
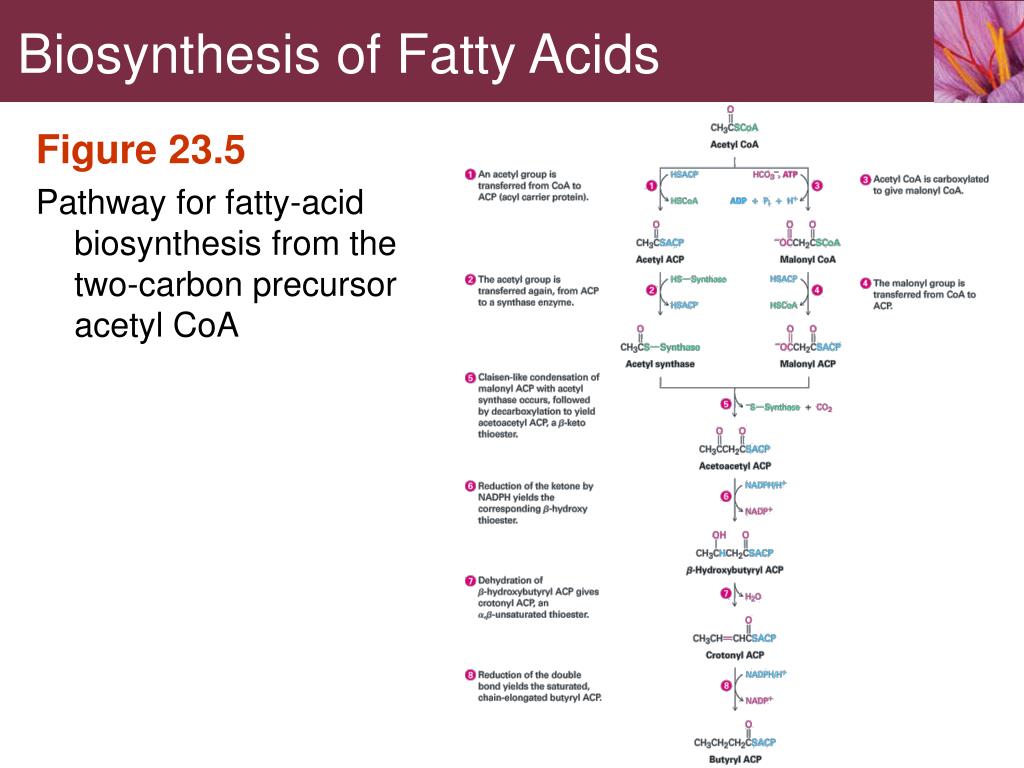
These effects show that the Marcus-like driving-force dependence for oxygen atom transfer from peroxides to the halides arises from the differing trends in hydration energies rather than in the intrinsic O–X – bond strengths. The first step in the reaction of a nucleophile with a carbonyl carbon atom is the formation of a new covalent bond, resulting in a species with four substituents around the central carbon atom (Figure 15 A). The strong decrease in E° from OCl – to OI – is seen to arise primarily from these differing trends in hydration energies rather than the gas-phase oxygen atom affinities of the halides. the sum of the potential energies of all the electron shells b. Similar trends are seen in the Gibbs energies of hydration. The images show spatial maps of the kinetic energy distribution (averaged over 1 ps after the energy plateaus post-reaction) for each atom of the metastable species 2b, 3c and 4c, as well as the. Both of these trends are contrary to expectations based on simple models of ionic hydration. It is found that the hydration enthalpies are more exothermic for the OX – species in comparison to their X – congeners, and it is found that the hydration enthalpies are approximately constant on progressing from OCl – to OI –. Experimental data are used to calculate conventional and absolute hydration enthalpies for OCl –, OBr –, and OI –. Common health hazards found in construction.
denum are shown, together with those of the primarily-produced species. Herein is a dissection of the energetic contributions to a correlation between the rates and driving forces for oxygen atom transfer from three inorganic peroxides to the halides. The purpose for the Occupational Safety and Health Administration (OSHA) and its enforcement duty under law. The yield of the trapped hydrogen atom, H., produced in the 6 M sulfuric acid.


 0 kommentar(er)
0 kommentar(er)
